Introduction
Ammonia solution, scientifically termed ammonium hydroxide (NH₃·H₂O), is a aqueous solution of ammonia gas dissolved in water. This compound, often recognized by its sharp, pungent odor, is a cornerstone of modern chemistry and industry. Its versatility arises from its unique physical and chemical properties, which enable applications ranging from household cleaning to large-scale industrial processes. This article examines the intrinsic characteristics of ammonia solution, including its physical state, reactivity, and behavior under varying conditions, while also exploring its practical uses, safety considerations, and environmental implications.
Physical Properties of Ammonia Solution
Ammonia solution is a colorless liquid with a characteristic odor that is highly recognizable even at low concentrations. Its physical attributes are critical to its handling and utility:
-
Solubility and Miscibility:
Ammonia exhibits exceptional solubility in water, with one volume of water dissolving approximately 700 volumes of ammonia gas at standard temperature and pressure (STP). This miscibility is attributed to hydrogen bonding between ammonia molecules and water, facilitated by ammonia’s polar nature. The solution’s concentration is typically expressed as a percentage of ammonia by weight or molarity, with commercial grades ranging from 5% to 28% NH₃. -
Density and Boiling Point:
The density of ammonia solution decreases with increasing temperature and varies inversely with concentration. A saturated solution at 20°C has a density of approximately 0.88 g/cm³, lighter than water. Notably, pure ammonia gas liquefies at -33.34°C under atmospheric pressure, but in aqueous solution, its boiling point rises to around 37.7°C for a 28% solution, reflecting the solution’s colligative properties.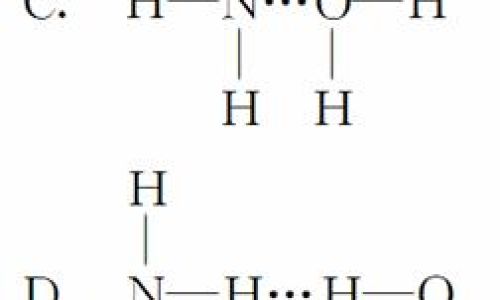
-
Volatility and Evaporation:
Ammonia solution is highly volatile, evaporating rapidly at room temperature. This property is exploited in refrigeration systems, where it acts as a refrigerant, and in analytical techniques requiring rapid solvent removal. -
pH and Basicity:
As a weak base, ammonia solution partially dissociates in water:
[
/text{NH}_3 + /text{H}_2/text{O} /rightleftharpoons /text{NH}_4^+ + /text{OH}^-
]
The equilibrium yields a pH between 10 and 12 for diluted solutions, making it mildly corrosive but less aggressive than strong bases like sodium hydroxide.
Chemical Properties and Reactivity
The chemical behavior of ammonia solution stems from its ability to act as a Brønsted-Lowry base, a nucleophile, and a ligand in coordination complexes:
-
Acid-Base Reactions:
Ammonia reacts vigorously with acids to form ammonium salts. For example, with hydrochloric acid:
[
/text{NH}_3 + /text{HCl} /rightarrow /text{NH}_4/text{Cl}
]
This reaction is exothermic and forms the basis for ammonia’s use in neutralizing acidic wastes. -
Reactions with Metals:
Ammonia solution reacts with certain metals, particularly those like copper, zinc, and nickel, to release hydrogen gas and form metal ammine complexes:
[
2/text{NH}_3 + 3/text{Cu} /rightarrow 3/text{Cu(NH}_3/text{)}_2^+ + 2/text{e}^- + /text{H}_2/uparrow
]
This reactivity is harnessed in metallurgical processes and electroplating. -
Complexation and Coordination Chemistry:
Ammonia acts as a ligand, donating lone pairs to form stable complexes with transition metals. The classic example is the formation of hexaaquacopper(II) ions in the presence of excess ammonia:
[
/text{Cu(H}_2/text{O)}_6^{2+} + 4/text{NH}_3 /rightarrow /text{Cu(NH}_3/text{)}_4^{2+} + 6/text{H}_2/text{O}
]
This property is pivotal in analytical chemistry for metal ion detection and separation. -
Thermal Decomposition:
Heating ammonia solution drives off ammonia gas, a principle utilized in its purification and concentration. At elevated temperatures, it decomposes entirely into nitrogen and hydrogen:
[
2/text{NH}_3 /xrightarrow{/Delta} 3/text{H}_2 + /text{N}_2
]
Industrial and Household Applications
The diverse reactivity of ammonia solution underpins its widespread use across sectors:
-
Cleaning Agents:
Ammonia’s ability to dissolve grease, oils, and proteins makes it a staple in household cleaners. Its low cost and efficacy against organic stains have cemented its role in products like glass cleaners and oven sprays.
-
Fertilizer Production:
Ammonia is a precursor to nitrogen-based fertilizers, including urea and ammonium nitrate. The Haber-Bosch process, which synthesizes ammonia from nitrogen and hydrogen, sustains global agriculture by providing essential nutrients to crops. -
Textile Industry:
In dyeing and printing, ammonia solution regulates pH and swells cellulose fibers, enhancing dye penetration. It is also used in mercerization, a process that strengthens cotton fabrics. -
Food Industry:
As a leavening agent, ammonia carbonate decomposes upon heating to release carbon dioxide, aerating baked goods. It also adjusts pH in cheeses and chocolates, ensuring proper texture and flavor development. -
Pharmaceuticals:
Ammonia solution is used in manufacturing antiseptics, cough syrups, and certain antibiotics. Its role in synthesizing APIs (active pharmaceutical ingredients) underscores its biomedical significance.
Laboratory Applications
In research settings, ammonia solution serves multiple functions:
-
Buffer Solutions:
Ammonia-ammonium chloride buffers maintain stable pH levels in biochemical assays, particularly those involving enzymes sensitive to acidity. -
Spectroscopy:
As a solvent for infrared (IR) spectroscopy, ammonia’s low volatility and transparency in the IR region facilitate the analysis of polar compounds. -
Crystal Growth:
Controlled evaporation of ammonia solutions aids in growing single crystals of metal complexes, crucial for X-ray crystallography.
Safety and Handling Precautions
Despite its utility, ammonia solution poses significant risks:

-
Toxicity:
Inhalation of ammonia vapors irritates respiratory tracts, while ingestion causes severe burns. Prolonged exposure may lead to pulmonary edema or chemical pneumonia. -
Corrosive Nature:
Contact with skin or eyes results in alkaline burns. Protective gear, including gloves, goggles, and face shields, is mandatory during handling. -
Reactive Hazards:
Mixing ammonia with bleach (sodium hypochlorite) generates toxic chloramine vapors:
[
2/text{NH}_3 + /text{NaOCl} /rightarrow /text{NaCl} + /text{N}_2/text{H}_4 + /text{H}_2/text{O}
]
Such reactions necessitate strict segregation of chemicals. -
Ventilation:
Fume hoods or adequate airflow prevent vapor accumulation, reducing inhalation risks.
Environmental Impact and Disposal
Ammonia’s ecological footprint extends to aquatic systems, where excess nitrogen fuels eutrophication, depleting oxygen and suffocating marine life. Industrial discharges are regulated to limit ammonia concentrations in wastewater. Proper disposal involves neutralization with acids before release, adhering to environmental guidelines.
Conclusion
Ammonia solution exemplifies the interplay between a compound’s properties and its applications. From its role in sustaining agricultural output to its presence in everyday cleaning products, its basic nature, reactivity, and versatility render it indispensable. However, its hazards demand rigorous safety protocols and environmental stewardship. As industries evolve, balancing ammonia’s benefits with sustainable practices remains paramount, ensuring its continued relevance in a resource-conscious world.
Word Count: 1,829+






0 comments